on the July 11, 2024

By binding to their receptors, intercellular communication molecules of the TGF-beta family can have agonist or antagonist effects, effects that are essential for the establishment of the dorsal and ventral regions of the embryo. In a paper published in the journal PLOS Biology in June 2024, Thierry Lepage's team at the Institut de Biologie Valrose reveals that, in the case of the antagonistic sea urchin TGF-beta PANDA, this dichotomy is based on the nature of a single amino acid in the interaction domain of the ligand to its receptor.
Panda: a TGF-beta with an enigmatic mechanism of action
In sea urchins, as in mammals, the spatial restriction of TGF-beta Nodal expression is the key element that breaks the radial symmetry of the embryo and allows the establishment of the dorso-ventral axis. This spatial restriction requires the localized production of TGF-beta family proteins which function as antagonists, binding to the same receptor as Nodal and blocking its signaling pathway and expression. In mice, the Lefty gene product acts as the first Nodal antagonist, restricting its expression. In sea urchins, on the other hand, it is a maternal factor (present from the egg stage) called Panda (Paracentrotus Anti Nodal Dorsalizing Activity) which acts as a Nodal antagonist to limit its expression, with Lefty intervening only later in the process. But the molecular basis of Panda's antagonistic activity was not understood.
The agonist/antagonist nature of TGF-beta may be encoded at the level of a single amino acid
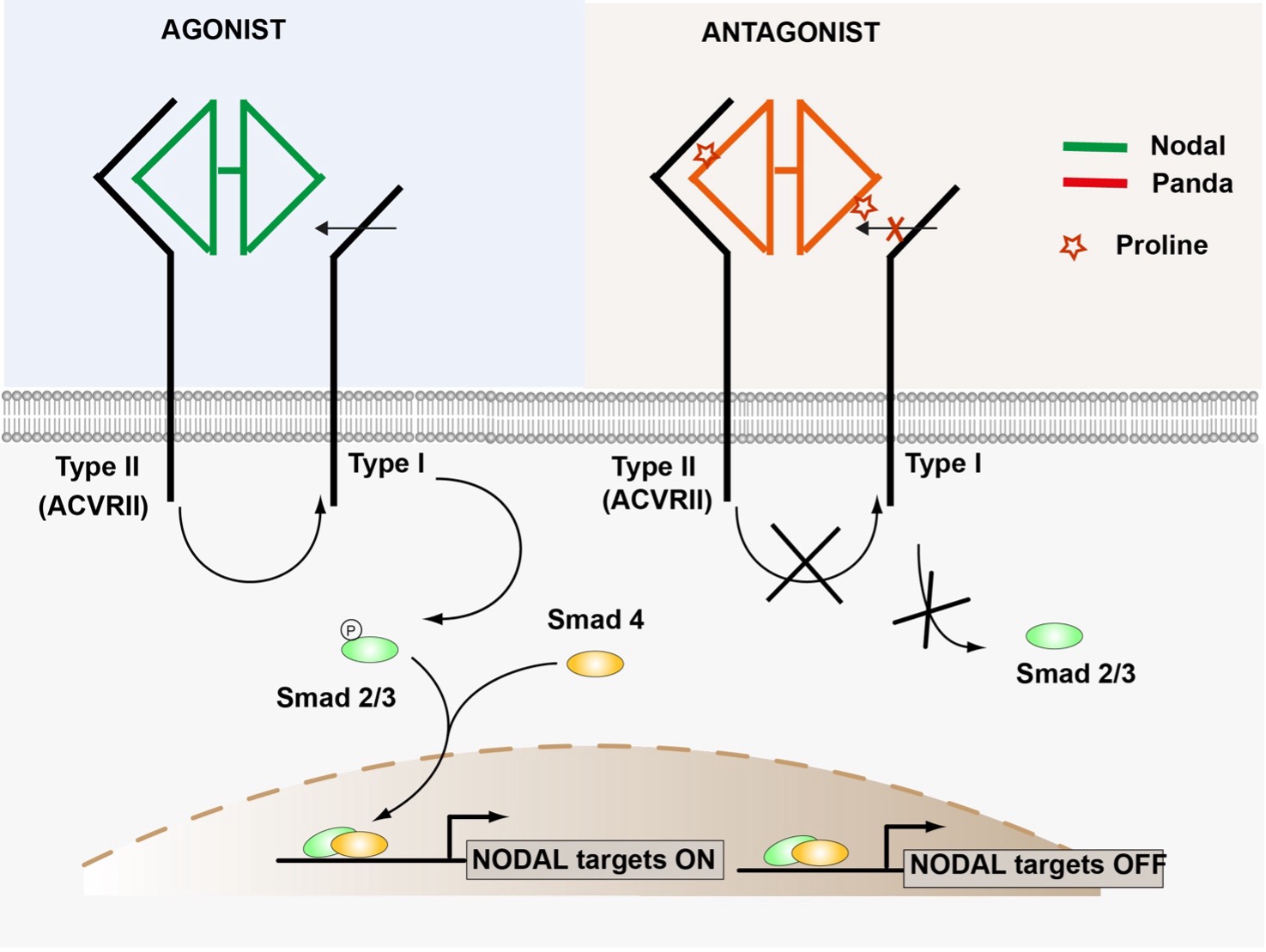
The work of a researcher at the Institut de Biologie Valrose lifts part of the veil on the mechanism by which Panda acts as a Nodal antagonist. First, the researchers discovered that Panda strongly inhibits the activity of ACVRII, the receptor to which Nodal binds, in keeping with its antagonist role. The scientists then focused on the region of Panda involved in binding to the ACVRII receptor. By analyzing the sequence alignments of various TGF-beta factors, they made an unexpected observation: in the sequences of all Panda and Lefty factors from different species, i.e. in virtually all TGF-beta that act as antagonists, an amino acid called proline is present in a central position in the region involved in interaction with the ACVRII receptor. In contrast, in all other TGF-beta agonists (the majority of BMPs, Nodal, Activin, GDF1-3 etc.), this amino acid is either serine or threonine. The researchers then replaced the proline amino acid with a serine within the Panda sequence. This replacement proved sufficient to radically alter Panda's activity on Nodal, from that of an antagonist favoring the acquisition of a dorsal identity by the cells, to that of an antagonist restricting the acquisition of a dorsal identity by the cells. identity by restricting nodal expression, to that of a Nodal agonist, promoting the acquisition of a ventral identity by cells by stimulating nodal expression.
The amino acid proline in the interaction domain between Panda and ACVRII is thought to act as a molecular switch on receptor activity.
These results show that changing a single amino acid can dramatically alter TGF-beta PANDA activity. They suggest that the agonist/antagonist nature of PANDA, and probably that of other members of the TGF beta family, is largely determined by a single amino acid in the region where the ligand interacts with the ACVRII receptor. This amino acid acts as a molecular switch on ACVRII activity: it activates ACVRII when it is a serine (agonist function), and inhibits it when it is a proline (antagonist function). These findings suggest that it may be possible not only to predict the agonist or antagonist activity of aa TGF-beta, but also to convert Lefty family antagonists into agonists such as Nodal, and Nodal/Activin family agonists into antagonists such as Leftys, by modifying the nature of the amino acid present within the ACVRII receptor-binding motif.
- Publication title and authors
-
Viswanathan PK, Chessel A, Molina MD, Haillot E, Lepage T (2024) Maternal TGF-β ligand Panda breaks the radial symmetry of the sea urchin embryo by antagonizing the Nodal type II receptor ACVRII. PLoS Biol 22(6): e3002701. https://doi.org/10.1371/journal.pbio.3002701
- Contact
-
Researcher :
Thierry Lepage, DR1 CNRS, http://ibv.unice.fr/EN/equipe/lepage.php
Thierry.LEPAGE@univ-cotedazur.fr (04 89 15 08 30)
Affiliation: Université Côte d'Azur / Institut de Biologie Valrose - CNRS - Inserm - France
iBV Scientific Communication: Nathalie Billon (nathalie.billon@univ-cotedazur.fr)



