from April 29, 2024 to September 30, 2024
With more than 10,000 people waiting for an organ transplant in France in 2023, the shortage of donations is prompting research to find other solutions.
For skin grafts, xenotransplantation could make it possible to treat even patients from whom healthy skin cannot be harvested. National Cancer Institute/Unsplash, CC BY
Grafting pig skin to heal wounds: a promising avenue
With more than 10,000 people waiting for an organ transplant in France in 2023, the shortage of donations is prompting research to find other solutions. Xenografts, which involve transplanting an organ from a donor whose biological species is different from that of the recipient, represent a promising avenue. The pig is considered to be the donor species of choice, due to the many physiological and morphological similarities between human and pig organs.
Recent advances include a kidney transplant in a brain-dead patient in 2023, and a heart transplant in a living American patient ineligible for a human transplant. But xenotransplantation is also an avenue for reconstructive surgery, to provide skin grafts.
Hope for repairing the most difficult wounds
While xenografts for organs such as the liver, kidney or heart can help to alleviate the shortage of grafts, their application in plastic surgery presents other challenges. This applies in particular to the treatment of so-called complex wounds, serious wounds that cannot be treated using simple techniques, without damaging the patient's healthy tissues. The economic and social impact of treating these wounds is rapidly increasing, due to rising healthcare costs and an ageing population.
These complex wounds occur in a wide variety of conditions: limb fractures, removal of severe and extensive skin cancers, wounds associated with vascular and neurological disorders in diabetic patients... They sometimes expose so-called "noble" structures such as bone, tendons and vessels to the open air. In the worst cases, they can lead to amputation of a limb or to generalized infection originating in the wound, which can result in death.
Avoiding graft rejection
Sometimes, the only solution for treating these wounds and avoiding complications is to cover them with tissue taken from the patient himself, called flaps. These flaps involve removing skin from healthy areas, which can have serious adverse consequences (reopening of the wound, loss of muscle function, nerve damage, pain, etc.). The patient may also lack healthy tissue harvesting areas, with the impossibility of taking tissue adapted to the wound to be covered in the case of skinny, burned or multi-operated patients. Using tissue flaps from pigs in xenografts could be a solution to these problems.
However, the use of xenografts is limited by interspecies immunological barriers. In the human bloodstream, antibodies are responsible for identifying non-human markers, known as xenoantigens, present on the surface of pig cells. This immune reaction is responsible for hyperacute rejection, which inexorably leads to graft loss within minutes.
Replacing animal cells with patient cells
One way of avoiding this immune reaction is to decellularize and then recellularize grafts. Organ decellularization consists in producing a cell-free (or acellular) matrix, retaining the original shape of a donor patient's or animal's organ, but consisting solely of the connective tissue that structures organs. Decellularization therefore removes the donor cells, while preserving the shape and nutrient environment for the cells, by treating the tissue or organ with detergents. As it is cell-free, this matrix does not cause rejection if transplanted into a recipient patient.
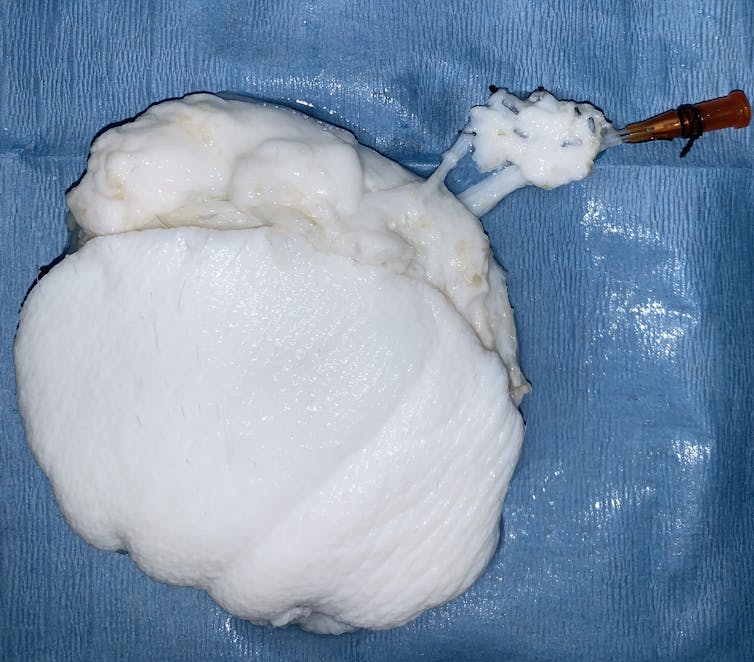
Patient cells can then be grown on this matrix prior to transplantation: this is known as recellularization. These recellularized matrices can then be transplanted to the recipient to restore, maintain or improve organ function, or cover a wound.
These recellularized matrices are recognized by the patient's body as part of "self", so that it does not reject them. While a number of simple, decellularized skin substitutes and dermal matrices have been produced and marketed (porcine heart valves, bovine artificial dermis...), no more elaborate graft has ever been integrated into a recipient patient, as the matrices in this case require this recellularization stage.
Advances, but no human attempt yet
While decellularization and recellularization have shown great potential in the transplantation of organs such as liver, kidney, heart or lung, their application to tissue flaps has only recently been studied. With my team, we have developed and optimized decellularized skin flap matrix models in pigs. All surgical procedures have been approved by the local ethics committee.
Skin flaps were harvested from live, anesthetized pigs in the operating room. These flaps were then perfused with a specific detergent at different concentration levels. We have shown that it is necessary to keep this concentration low to maintain a nutritious environment, essential for welcoming cells back. If the detergent concentration is too high, the matrix becomes toxic for the cells, which do not survive.
We verified that these skin matrices preserved the basic mechanical and chemical properties of porcine skin. Proteins and growth factors were present in sufficient quantities in the matrices for cells to live. We have finally demonstrated the possibility of recellularizing acellular matrices. However, we still need to optimize the recellularization strategy, so as to be able to deposit very large numbers of cells on the matrices.
Such a treatment for pig flap grafts, which does not induce rejection in humans as it contains the patient's own cells to be treated, would free us from the complications associated with harvesting tissue from the patient's own healthy skin. This technology would also solve the problem of the absence of a healthy donor site. Further research is crucial if we are ever to achieve xenotransplantation of human skin flaps without immediate rejection.![]()
Elise Lupon, PhD student in clinical and therapeutic research, Université Côte d'Azur
This article is republished from The Conversation under Creative Commons license. Read theoriginal article.
Doctoral student in clinical and therapeutic research, Université Côte d'Azur
THE CONVERSATION
Since January 2022, Université Côte d'Azur, through its Science and Society department, has been a member of the online news medium "The Conversation". The aim is to showcase the work of our research teams in the service of enlightened, reliable information that contributes to civic debate.
The Conversation is an online medium and a non-profit association. Its model of collaboration between experts and journalists is unique: to share knowledge, making the voice of researchers heard in civic debate, and enlightening the news with reliable, research-based expertise.
theconversation.com
SUBSCRIBE NOW!
To keep up with The Conversation's experts on a daily basis, subscribe to our newsletter.
https://theconversation.com/fr/newsletters/la-newsletter-quotidienne-5



