on the November 6, 2023
Two teams from the Valrose Institute of Biology (Université Côte d’Azur, CNRS, Inserm) have identified an essential factor in the initiation of ovarian development. This factor is a splice variant of the Wt1 gene, which plays a key role in sex determination. This work was published in the prestigious journal Science on November 2, 2023.
In most mammals, sex is determined at fertilization. The mother transmits the X sex chromosome while the father transmits an X or Y chromosome. Thus the XX embryo becomes a female while an XY individual becomes a male through the process called “sex determination”. This process allows the undifferentiated gonad to develop into an ovary or testis respectively, thus initiating all of the individual's sexual development.
In XY individuals, the testicular differentiation program is governed by the Sry gene, located on the Y chromosome. Sry activates different target genes including Sox9, which is necessary for the differentiation of male support cells, Sertoli cells. These cells are not only necessary for the development of the testicle but also for the establishment and maintenance of spermatogenesis.
In females, the maintenance of ovarian differentiation requires Rspo1, an activator of the WNT/ß-catenin signaling pathway and the transcription factor FOXL2. However, the mechanism of initiation of the ovarian differentiation program remained elusive until now. Analysis of patients affected by differences in sexual development suggests that one of the genes important for sex determination is the WT1 (Wilms’ tumor suppressor) gene. During the expression of this gene, the alternative splicing between the last two exons generates two variants, one which lacks the three KTS amino acids (lysine, threonine and serine) and one which does not, named –KTS and +KTS respectively. Mutations leading to an imbalance in the production of these variants are responsible for Frasier syndrome, which is characterized by the feminization of the genitalia of XY patients.
To analyze the role of these isoforms +KTS and -KTS in the development of the gonad, Elodie Gregoire, of the "Determination of sex and fertility" team at the Valrose Institute of Biology, examined mouse models expressing only one of these two isoforms and showed that:
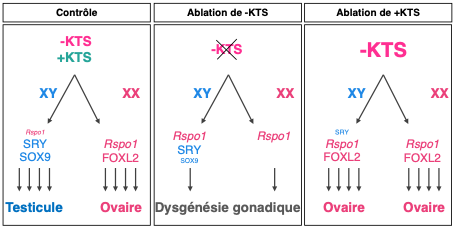
• the absence of -KTS prevents differentiation of female support cells, granulosa cells and any ovarian development. Cells remain blocked at a progenitor cell stage.
• overexpression of -KTS activates ovarian development and prevents activation of the Sry gene in the XY gonad. This gonad becomes an ovary rather than a testicle (Figure 1).
These data show that the –KTS isoform is essential and indispensable for ovarian development, making this Wt1 variant a key factor in female sexual differentiation.
schema
Figure 1. Model of support cell differentiation in wild-type and KTS mutant gonads: The absence of -KTS in the gonads induces gonadal dysgenesis whereas its overexpression leads to ovarian differentiation regardless of XX or XY genetic sex.
• Image copyright: Elodie Gregoire
• Figure copyright: Elodie Gregoire and Marie-Christine Chaboissier
- More information :
-
The −KTS splice variant of WT1 is essential for ovarian determination in mice
Gregoire E.P., De Cian M.C., Migale R., Perea-Gomez A., Schaub S., Bellido-Carreras N., Stevant I., Mayere C., Neirijnck Y., Loubat A., Rivaud P., Llorian Sopena M., Lachambre S., Linssen M.M., Hohenstein P., Lovell-Badge R., Nef S., Chalmel F., Schedl A. et Chaboissier M.C.
Science 2023, sous presse - Contact :
-
Marie-Christine Chaboissier - Directrice de recherche CNRS
marie-christine.CHABOISSIER@univ-cotedazur.fr
Elodie Grégoire - Ingénieure d’étude INSERM
Elodie.gregoire@univ-cotedazur.fr
Institut de Biologie Valrose (iBV)
Université Cote d’Azur – Parc Valrose – 06108 Nice



